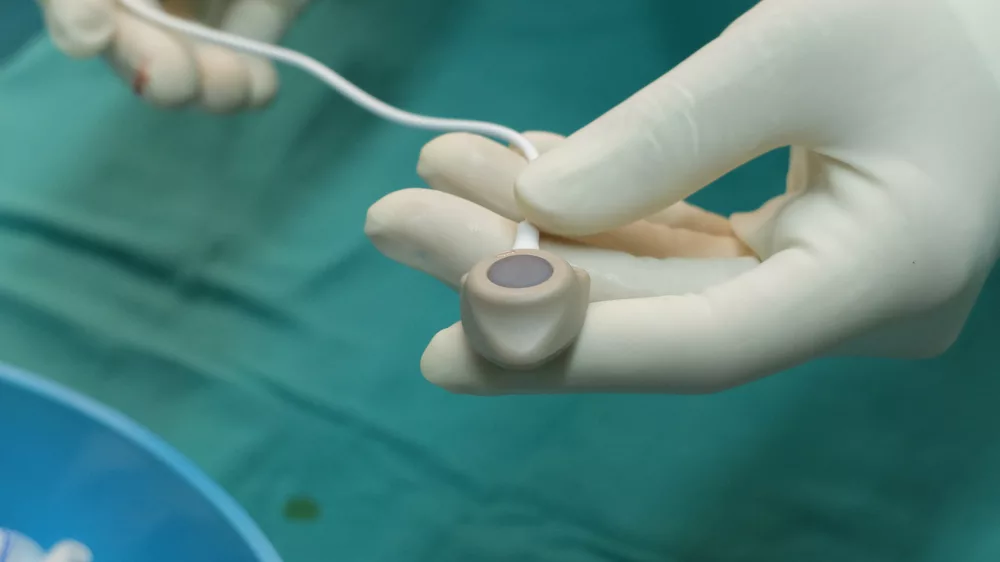

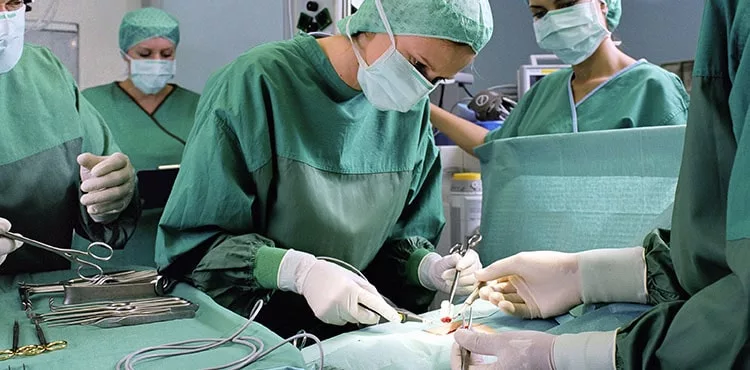
The Bard PowerPort Catheter is an implantable device produced by Bard Access Systems Inc. and C.R. Bard Inc that is designed to deliver medications and fluids directly to the bloodstream.
Due to the high concentration of Barium Sulfate, the device can quickly lose its integrity once implanted, possibly leading to fracturing, cracking, or migration of the device.
These complications may lead to serious and life-threatening injuries that may require medical or even surgical intervention. Side effects of a Bard PowerPort Catheter failure include:
Victims of Bard PowerPort Catheter failures were not notified that they were at greater risk due to these devices. Bard Access System Inc. has received multiple adverse event reports for years and still continues to allow these products to be on the market. Litigation is in its beginning stages and many victims have already begun filing suits.




We prosecute pharmaceutical and medical device injuries. Every day, we…

Auto accidents occur on Texas roads approximately every 67 seconds,…
The position will involve litigating product liability cases throughout all phases, from filing to trial. The job requires strong legal research and writing skills and a solid grasp of civil procedure.
Responsibilities
Responsibilities