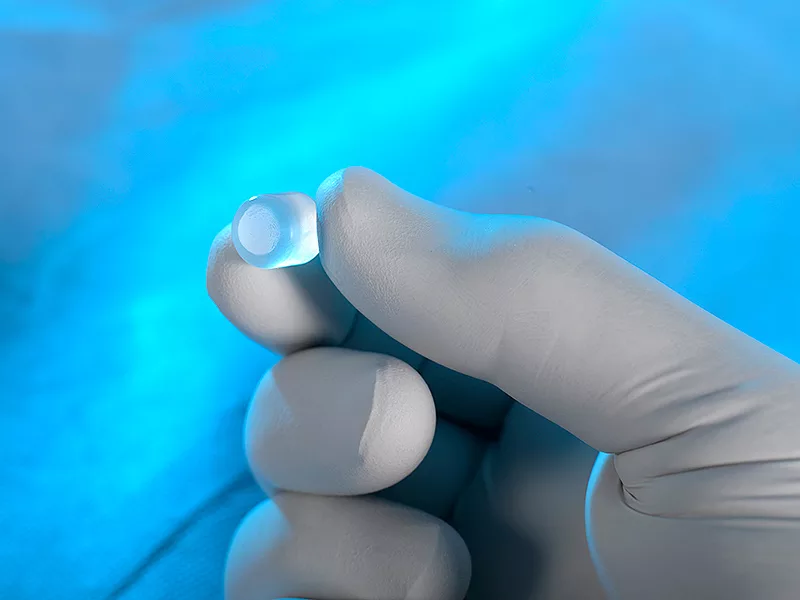
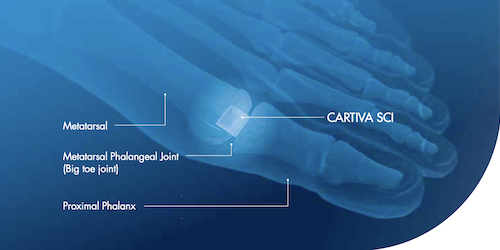


The Cartiva implant is a molded cylindrical device used to treat arthritis within the big toe, a condition impacting over 2 million people in the United States. This medical device was recently developed as an alternative to arthrodesis, or a fusion surgery. The implant itself is comprised of a material called polyvinyl hydrogel meant to mimic cartilage within the metatarsophalangeal joint.
Recent evidence shows that the implants are defectively designed and are prone to catastrophic failures leading to significant pain, deformity, bone loss, and the need for additional surgical intervention, usually in the form of a fusion surgery. A new study shows that the implant has a staggering high failure rate: “as many as 64% of individuals who received a Cartiva implant for hallux rigidus experienced failure within four weeks of surgery, and the failure rate increased to 79% after 19 months.”
The Cartiva toe implant was manufactured and sold by Styker, which failed to adequately study potential adverse effects and failed to adequately warn the public of the actual risks involved in having the surgery performed. As a result, patients have experienced severe toe pain, fractured bones, loss of range of motion, deformity, and more.
If you have had the Cartiva toe implant surgically installed and subsequently experienced pain or complications, please contact Nachawati Law Group today for a free consultation.




We prosecute pharmaceutical and medical device injuries. Every day, we…

Auto accidents occur on Texas roads approximately every 67 seconds,…
The position will involve litigating product liability cases throughout all phases, from filing to trial. The job requires strong legal research and writing skills and a solid grasp of civil procedure.
Responsibilities
Responsibilities