


The FDA Identified the Following Possible Injuries
- Hypoglycemia — Low Blood Glucose
- Hyperglycemia — High Blood Glucose
- Loss of Consciousness
- Nausea and Vomiting
- Shortness of Breath
- Weakness
- Seizure
- Coma
- Death
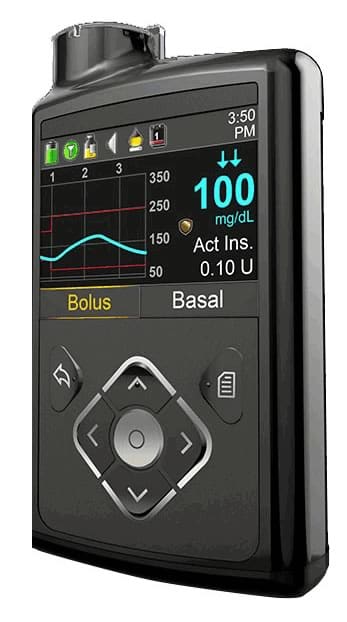
On Nov. 21, 2019, Medtronic Inc. initiated a recall of two insulin pump models intended to be used by individuals with Type 1 diabetes. The recall includes over 300,000 individual pumps. The U.S. Food and Drug Administration (FDA) identified the recall as a "Class I recall, the most serious type of recall" because the use of "these devices may cause serious injuries or death." Medtronic has stated the pumps have already been involved in 26,421 complaints, including 2,175 injuries and one death.
Medtronic has recalled the Minimed 630G and Minimed 670G because these pumps have a missing or broken "retainer ring." This retainer ring helps lock the insulin cartridge in the pump's reservoir compartment. Medtronic stated that if the cartridge is not locked firmly, the device may deliver the wrong amount of insulin to the user.
Minnesota-based Medtronic Inc. is one of the world's largest medical device corporations and the largest manufacturer of insulin pumps and infusion sets. If the pump's retainer ring is broken or missing, and the device delivers too much or too little insulin to the user, severe, life-threatening consequences may occur.
Any person age 16 or older with Type 1 diabetes that uses a Model 630G distributed between September 2016 to October 2019, or a Model 670G distributed between June 2017 to August 2019, may be at risk.




We prosecute pharmaceutical and medical device injuries. Every day, we…

Auto accidents occur on Texas roads approximately every 67 seconds,…
The position will involve litigating product liability cases throughout all phases, from filing to trial. The job requires strong legal research and writing skills and a solid grasp of civil procedure.
Responsibilities
Responsibilities