1.What is Elmiron?
Elmiron is the brand name for pentosan polysulfate, a drug first approved in 1996 that is used to treat interstitial cystitis, also known as bladder pain syndrome. Interstitial cystitis is estimated to affect more than 1 million individuals in the United States. Janssen Pharmaceuticals manufactures and distributes Elmiron, which is licensed by Teva Pharmaceuticals.
2.What is Wrong with Elmiron?
A number of studies have shown that Elmiron may cause a serious eye condition called maculopathy—the degeneration of the macula (the central part of the retina). Maculopathy is characterized by blurry, blank, or dark spots in the center of vision, difficulty reading, colorblindness, and difficulty adjusting to low light or changes in lighting.
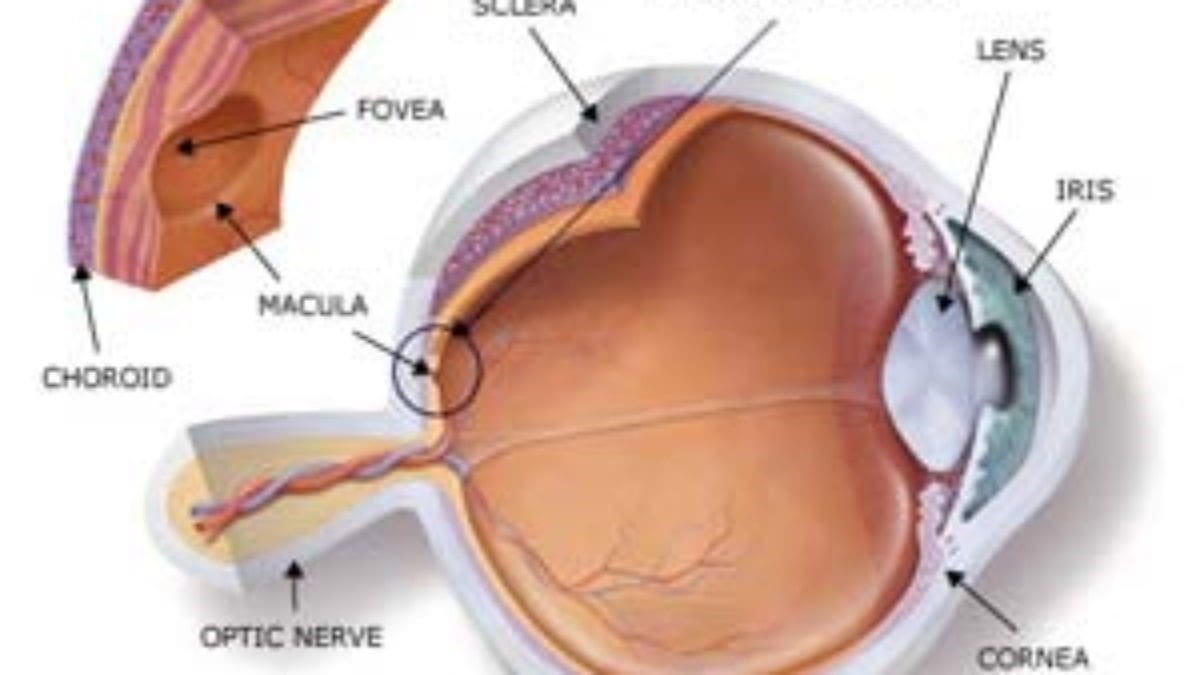
In November 2018, a study published in Ophthalmology, the journal of the American Academy of Ophthalmology, demonstrated the association between regular use of Elmiron and maculopathy. William A. Pearce et al., Pigmentary Maculopathy Associated with Chronic Exposure to Pentosan Polysulfate Sodium, 125 Ophthalmology 1793 (2018). A follow-up study in 2019 published in Ophthalmology further established this relationship. Adam M. Hanif, Strength of Association between Pentosan Polysulfate and a Novel Maculopathy, 126 Ophthalmology 1464 (2019).
A larger and more detailed 2019 study showed that 7 years after using Elmiron, a person’s risk of developing maculopathy is significantly increased (OR=1.41, 95% CI 1.09 to 1.83, p=0.009). Several other recent studies confirmed this strong association between Elmiron use and maculopathy. There also appears to be some dose-dependence, with higher amounts of usage correlating with higher levels of damage. It is not currently known whether the vision damage caused by Elmiron is permanent or progressive, whether instead stopping use of Elmiron limits the amount of damage, or whether the damage is reversible.
Although Elmiron’s labeling has been updated 5 times since it originally went on the market, the labeling was silent about Elmiron’s maculopathy risks until June 16, 2020, when the following black box warning was added:
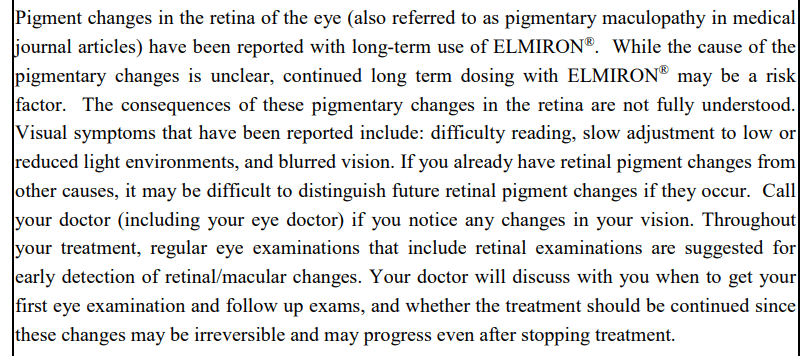
This label change came 8 months after Health Canada (the department of the Canadian Government responsible for national health policy) mandated that pigmentary maculopathy be added to the “Warnings and Precautions” included on Elmiron labeling.
3.What are We Looking for in Elmiron Cases?
a.) At least 1 year of use with positive retinal damage, or at least 3 years of use with substantial vision loss
b.) Diagnosis (any of the following):
- Maculopathy
- macular degeneration
- drusen
- choroidal neovascularization
- retinal toxicity
c.) Injuries (any of the following):
- Central vision loss
- Blurred, dimmed, distorted, or spotty vision
- Difficulty reading
- Difficulty adjusting/prolonged adjustment to low light or changes in lighting
- Pain in the eye
- Colorblindness